Over the summer, my then-pregnant friend asked for my opinion about umbilical cord blood banking, naturally sending me into a world of fascinating biology, cutting edge medicine and some ethical quandaries.
If you can afford the $1000-2000 processing fee and at least $100 a year to store the blood, banking seems like a no-brainer. “You never know,” rings in the backs of many expecting parents’ minds as the one-time opportunity approaches. But there is more to consider than price. The biology behind the technique and the currently available applications of frozen cord blood may influence one’s decision about whether to bank, and also how and where to do it.
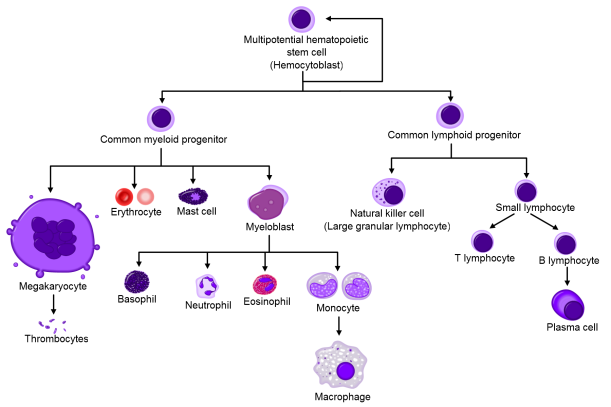
Cord blood contains a high frequency of hematopoietic stem cells, which can differentiate into any kind of blood cell. They can mature into megakaryocytes that make platelets, red blood cells, or immune cells like B cells or eosinophils. We all carry these stem cells throughout our lives, mainly in our bone marrow, and they produce cells that periodically replace blood cell populations.
Cancers rising from white blood cells (like lymphoma or leukemia) and genetic defects interfering with the production of any kind of blood cell can conceivably be addressed by resetting the whole system with a bone marrow (or stem cell) transplant. Transplants using donated bone marrow have been used to do just this for about 50 years. In the late 1980s, it became clear that cord blood stem cells could do the same with some distinct advantages.
For one thing, collecting cord blood is much less difficult and invasive compared to harvesting bone marrow. Bone marrow donation involves anesthesia and a very large needle stuck directly into the bone. Stem or progenitor cells can also be separated from adult blood through a process called apheresis, but it is no cakewalk, especially compared to harvesting cord blood, which simply involves injecting a needle into the cord after it’s been cut.
Donor matching is also more flexible for cord blood. To avoid graft rejection, stem cell (and all organ) donors and recipients are matched for proteins expressed on the surface of immune cells. If they don’t match, the T cells in the donated transplant may attack the tissues of the recipient. T cells found in cord blood respond with less gusto and there are higher frequencies of T cell subsets that control the immune response called T regulatory cells. This means there’s a lower chance of the donor immune system harming the recipient.
Given these advantages, is banking worthwhile? It depends on what you hope to get out of it. When you think about storing a baby’s cord blood, you may think it’s for the sake of that particular child. The truth is, the stem cells in that kid’s blood are more likely to be useful for someone else. That was the case of the first ever cord blood transplant performed in 1988. A five year-old boy with a rare genetic disease called Fanconi Anemia received cord blood cells from his newborn sister. At the time, the boy’s white blood cell counts were dropping because inherited defects in a DNA repair pathway made it impossible for his bone marrow to produce healthy blood cells quickly enough. His own cord blood, of course, would have been useless because the same genetic defect would manifest again. Today, that patient is a grown man, but he has a female blood and immune system thanks to his sister.
Cases covered in the news about cancer patients cured because of cord blood transplants are usually about patients who received donated cord blood from public banks. In fact, their own stem cells would not have worked. In such cases, the transplant is an imperfect match on purpose so that the new immune system will attack cancer cells that the old immune system was blind to. This is typically done for blood cancers like leukemia. A transplant of a close or perfect match is desirable for patients whose bone marrow is depleted as a side effect of chemotherapy and/or irradiation for other types of cancer. However, stem cells for this kind of transplant can also be harvested from one’s own bone marrow or blood before beginning treatment.
photo credit: Banc de Sang i Teixits via photopin
Even if the chances of using one’s own cord blood are remote, it may be desirable to store it in case a family member could use it. There are a couple of caveats to consider, however, before committing to private banking. First, it’s been estimated that at least 70% of adult recipients need two units of cord blood to successfully reinstate a new blood and immune systems That means it’s likely that even if you do save your baby’s cord blood, it may not be enough if he or she needs it as an adult. This may change in the future; a study published last week in Science found that a drug compound called UM171 kept human cord blood stem cells “immature” while allowing them to expand. There are also several clinical trials underway that will test whether expanding cord blood progenitors through other means can reduce the number of units needed and increase transplant success.
The second caveat is a little bit more about logistics and politics than science. Right now, only 10% of collected cord blood meets the standards required for transplantation. These standards included how many cells are present, how many cells survived and whether the blood was collected, shipped and frozen properly. (For an interesting glimpse at what could go wrong, check out this Wall Street Journal article). And the way cord blood units are handled and stored is only regulated by the Food and Drug Administration if they are stored in public banks. That is not to say that there are no good and reputable private banks. It is, however, important to recognize that private banking requires lots of research and care when choosing a company.
I mentioned that the chances of performing a cord blood transplant on the original donor are not very high given the current uses of cord blood stem cells—mainly to replace blood stem cells in the bone marrow. That is not the whole story though. There are clinical trials going on to test the therapeutic effects of cord blood stem cells for things like cerebral palsy, type I diabetes and even hearing loss. These studies are based on observations suggesting stem cells found in cord blood can reduce brain damage after injury, but it’s not yet clear how. There are also other kinds of stem cells in cord blood that can differentiate into cells other than blood cells (pancreatic cells for example). There may still be a lot of untapped potential for cord blood. For many parents, that is enough reason to put their kids’ blood “on ice” and wait it out.
Sources:
Metheny L., Caimi P. & de Lima M. (2013). Cord Blood Transplantation: Can We Make it Better?, Frontiers in oncology, PMID: http://www.ncbi.nlm.nih.gov/pubmed/24062989
Gluckman E., Arleen D. Auerbach, Henry S. Friedman, Gordon W. Douglas, Agnès Devergie, Hélène Esperou, Dominique Thierry, Gérard Socie, Pierre Lehn & Scott Cooper & (1989). Hematopoietic Reconstitution in a Patient with Fanconi’s Anemia by Means of Umbilical-Cord Blood from an HLA-Identical Sibling, New England Journal of Medicine, 321 (17) 1174-1178. DOI: http://dx.doi.org/10.1056/nejm198910263211707
Wagner J.E. Should double cord blood transplants be the preferred choice when a sibling donor is unavailable?, Best practice & research. Clinical haematology, PMID: http://www.ncbi.nlm.nih.gov/pubmed/19959107
Fares I., Chagraoui J., Gareau Y., Gingras S., Ruel R., Mayotte N., Csaszar E., Knapp D.J.H.F., Miller P. & Ngom M. & Cord blood expansion. Pyrimidoindole derivatives are agonists of human hematopoietic stem cell self-renewal., Science (New York, N.Y.), PMID: http://www.ncbi.nlm.nih.gov/pubmed/25237102
Petrini C. (2014). Umbilical cord blood banking: from personal donation to international public registries to global bioeconomy., Journal of blood medicine, PMID: http://www.ncbi.nlm.nih.gov/pubmed/24971040